MCQ Questions for Class 10 Science Chapter 4 Carbon and Its Compounds
| CBSE Class 10 | SCIENCE |
|---|---|
| Question Type | MCQ |
| No. of Questions | 30+ |
| Useful for | Class 10 Studying Students |
Class 10 Science Chapter 4 Carbon and Its Compounds MCQs with Answers
1. The isomeric pair is
(a) ethane and propane
(b) propane and butane
(c) ethane and ethane
(d) butane and 2-methyl propane
Answer
(d)2. The structural formula of ethyl ethanoate

Answer
(b)3. Which of the following is used to oxidise ethanol to ethanoic acid?
(a) Alkaline KMnO4
(b) Conc. H2SO4
(c) Acidified K2Cr2O7
(d) All of above
Answer
(d)4. The compound which gives a brisk effervescence with sodium metal and not with sodium hydrogen carbonate is
(a) ethanol
(b) ethanoic acid
(c) both ethanoic acid and ethanol
(d) none of these
Answer
(a)5. Identify the product formed when methane reacts with chlorine in the presence of sunlight is
(a) C2Cl6
(b) CH3Cl
(c) CHCl4
(d) None of these
Answer
(b)6. Which is denatured spirit?
(a) ethanol only
(b) ethanol and methanol (50%)
(c) ethanol and methanol (5%)
(d) methanol only
Answer
(c)7. Drinking alcohol and driving may cause serious accidents. To discourage this, police randomly test drivers for alcohol using a breath analyser. The breath analyser works because
(a) Alcohol makes the breath dry and the machine registers moisture
(b) Alcohol makes the breath hotter which changes the machine reading
(c) Alcohol causes more saliva which the machine checks.
(d) Alcohol in the breath cause a chemical change registered by the machine.
Answer
(b)8. Tertiary butane gets oxidised with oxidising agents like alkaline KMNO4 to
(a) Isobutane
(b) Ter-butyl alcohol
(c) Secondary-propyl alcohol
(d) All of above
Answer
(b)9. According to IUPAC system, the correct name of the organic compound is
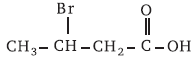
(a) 2-bromobutanoic acid
(b) 2-bromobutysis acid
(c) 3-bromobutanoic acid
(d) 3-bromo-2-hydroxybutan-2-one
Answer
(c)10. The substance not responsible for the hardness of water is
(a) sodium nitrate
(b) calcium hydrogen carbonate
(c) calcium carbonate
(d) magnesium carbonate
Answer
(a)11. The by product of soap is
(a) isoprene
(b) glycerol
(c) butene
(d) ethylene glycol
Answer
(b)12. Covalent compounds
(a) have high melting and boiling points
(b) are mostly soluble in water
(c) are formed between atoms of metals and non-metals
(d) are formed by the sharing of electrons in the bonding atoms.
Answer
(d)13. The heteroatoms present is CH3 – O – CH2 – CH2 (Br)
(a) oxygen
(b) carbon
(c) hydrogen
(d) bromine
Answer
(d)14. Vinegar is a solution of
(a) 30% – 40% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 15% – 20% acetic acid in water
Answer
(c)15. Which of the following can be used for the denaturation of ethyl alcohol?
(a) Methyl alcohol
(b) Pyridines
(c) Copper sulphate
(d) All of above
Answer
(d)16. Soaps are formed by saponification of
(a) alcohols
(b) glycosides
(c) simple esters
(d) carboxylic acids
Answer
(c)17. The correct electron dot structure of a water molecule is

Answer
(c)18. Acetic acid was added to a liquid X kept in a test tube. A colourless and odourless gas Y was evolved. The gas was passed through lime water which turned milky. It was concluded that:
(a) Liquid X is sodium hydroxide and the gas Y is CO2
(b) Liquid X is sodium carbonate and the gas Y is CO2
(c) Liquid X is sodium acetates and the gas Y is CO2
(d) Liquid X is sodium chloride and the gas Y is SO2.
Answer
(b)19. For gas welding used for welding broken pieces of iron, we normally use a mixture of
(a) Ethane and oxygen
(b) Ethene and oxygen
(c) Ethyne and oxygen
(d) Ethene and air
Answer
(a)20. Identify the compound that undergoes bromination reaction:

Answer
(d)21. Bromine reacts with saturated hydrocarbon at room temperature in the
(a) absence of sunlight
(b) presence of water
(c) presence of sunlight
(d) presence of hydrochloric acid
Answer
(c)22. The number of single and double bonds present in benzenes are
(a) 9 and 6
(b) 9 and 3
(c) 12 and 3
(d) 12 and 6
Answer
(b)23. Identify the correct way of numbering an organic compound (according to IUPAC)

Answer
(a)24. Identify the functional group present in the following compound

(a) aldehyde
(b) bromine
(c) carboxylic
(d) both bromine and carboxylic group
Answer
(d)25. The upper and lower homologue of C2H5OH are respectively
(a) methyl alcohol and butyl alcohol
(b) ethyl alcohol and propyl alcohol
(c) butyl alcohol and propyl alcohol
(d) propyl alcohol and methyl alcohol
Answer
(d)26. Which is not true about homologous series?
(a) They have same general formula.
(b) They differ from other by CH3 group.
(c) They have same functional group.
(d) They have same chemical properties.
Answer
(b)27. Name the following aromatic compound

(a) toluene
(b) aniline
(c) phenol
(d) furan
Answer
(a)28. Ethanoic acid was added to sodium carbonate solution and the gas evolved was tested with a burning splinter. The following four observations were reported. Identify the correct observation.
(a) The gas burns with pop sound and the flame gets extinguished
(b) The gas does not burn but the splinter burns with pop sound
(c) The flame extinguishes and the gas does not burn
(d) The gas burns with a blue flame and the splinter burns brightly
Answer
(c)29. Which of the following is not a straight chain?

Answer
(d)30. The general formula for alkanes is CnH2n+1–CHO. The value of ‘n’ for the first member.
(a) 1
(b) 0
(c) 0.5
(d) 1.1
Answer
(b)31. An organic compound ‘X’ has the molecular formula C3H6O2. It has a pleasant smell but does not turn blue litmus red. It has structural formula

Answer
(b)Related Posts
Category Lists (All Posts)
Select Category
All categories of this website are listed below with number of posts in each category for better navigation. Visitors can click on a particular category to see all posts related to that category.
- Articles (2)
- Full Form (1)
- Biography of Scientists (1)
- Biology (65)
- Blog Posts (35)
- Career Guidance (1)
- CBSE Class 10 Maths (77)
- CBSE Class 10 Science (187)
- Assertion Reason Questions for Class 10 Science (16)
- Case Study Questions for Class 10 Science (14)
- Evergreen Science Book Solutions for Class 10 (17)
- Extra Questions for Class 10 Science (23)
- HOTS for Class 10 Science (17)
- Important Questions for Class 10 Science (10)
- Lakhmir Singh Class 10 Biology Solutions (4)
- Lakhmir Singh Class 10 Chemistry Solutions (5)
- Lakhmir Singh Class 10 Physics Solutions (5)
- MCQ Questions for Class 10 Science (20)
- NCERT Exemplar Solutions for Class 10 Science (16)
- NCERT Solutions for Class 10 Science (15)
- Quick Revision Notes for Class 10 Science (4)
- Study Notes for Class 10 Science (17)
- CBSE Class 10 Social Science (45)
- CBSE CLASS 11 (1)
- CBSE Class 11 Chemistry (55)
- CBSE Class 11 Entrepreneurship (9)
- CBSE Class 11 Geography (48)
- CBSE Class 11 History (24)
- CBSE Class 11 Maths (63)
- CBSE Class 11 Physical Education (11)
- CBSE Class 11 Physics (154)
- Assertion Reason Questions for Class 11 Physics (15)
- Case Study Questions for Class 11 Physics (12)
- Class 11 Physics Study Notes (5)
- Concept Based Notes for Class 11 Physics (2)
- Conceptual Questions for Class 11 Physics (10)
- Derivations for Class 11 Physics (3)
- Extra Questions for Class 11 Physics (13)
- MCQ Questions for Class 11 Physics (16)
- NCERT Solutions for Class 11 Physics (16)
- Numerical Problems for Class 11 Physics (4)
- Physics Formulas for Class 11 (7)
- Revision Notes for Class 11 Physics (11)
- Very Short Answer Questions for Class 11 Physics (11)
- CBSE Class 11 Political Science (40)
- CBSE CLASS 12 (8)
- CBSE Class 12 Biology (27)
- CBSE Class 12 Business Studies (24)
- CBSE Class 12 Chemistry (82)
- Assertion Reason Questions for Class 12 Chemistry (15)
- Case Study Based Questions for Class 12 Chemistry (14)
- Extra Questions for Class 12 Chemistry (5)
- Important Questions for Class 12 Chemistry (15)
- MCQ Questions for Class 12 Chemistry (8)
- NCERT Solutions for Class 12 Chemistry (16)
- Revision Notes for Class 12 Chemistry (7)
- CBSE Class 12 Economics (19)
- CBSE Class 12 English (3)
- CBSE Class 12 Entrepreneurship (14)
- CBSE Class 12 Geography (18)
- CBSE Class 12 History (21)
- CBSE Class 12 Informatics Practices (29)
- CBSE Class 12 Maths (50)
- CBSE Class 12 Physical Education (32)
- CBSE Class 12 Physics (127)
- Assertion Reason Questions for Class 12 Physics (16)
- Case Study Based Questions for Class 12 Physics (14)
- Class 12 Physics Conceptual Questions (16)
- Class 12 Physics Discussion Questions (1)
- Class 12 Physics Latest Updates (2)
- Derivations for Class 12 Physics (8)
- Extra Questions for Class 12 Physics (4)
- Important Questions for Class 12 Physics (8)
- MCQ Questions for Class 12 Physics (14)
- NCERT Solutions for Class 12 Physics (18)
- Numerical Problems Based on Class 12 Physics (16)
- Physics Class 12 Viva Questions (1)
- Revision Notes for Class 12 Physics (7)
- CBSE Class 12 Political Science (33)
- CBSE Class 6 Maths (28)
- CBSE Class 6 Science (42)
- CBSE Class 6 Social Science (27)
- CBSE Class 7 (80)
- CBSE Class 7 Maths (33)
- CBSE Class 7 Science (54)
- CBSE Class 7 Social Science (31)
- CBSE Class 8 Maths (31)
- CBSE Class 8 Science (45)
- CBSE Class 8 Social Science (50)
- CBSE Class 9 English (2)
- CBSE Class 9 Maths (70)
- CBSE Class 9 Science (127)
- Assertion Reason Questions for Class 9 Science (16)
- Case Study Questions for Class 9 Science (15)
- Evergreen Science Book Solutions for Class 9 (15)
- Extra Questions for Class 9 Science (22)
- MCQ Questions for Class 9 Science (11)
- NCERT Solutions for Class 9 Science (15)
- Revision Notes for Class 9 Science (1)
- Study Notes for Class 9 Science (15)
- Topic wise MCQ Questions for Class 9 Science (2)
- Topicwise Questions and Answers for Class 9 Science (15)
- CBSE Class 9 Social Science (34)
- CHEMISTRY (8)
- Chemistry Articles (2)
- Daily Practice Problems (DPP) (3)
- Download Books (4)
- Editable Study Materials (8)
- Exam Special (6)
- H. C. Verma (Concepts of Physics) (13)
- ICSE Class 10 Biology (14)
- ICSE Class 10 Chemistry (6)
- ICSE Class 10 Maths (16)
- ICSE Class 10 Physics (25)
- ICSE Class 9 Maths (7)
- ICSE Class 9 Physics (10)
- IIT Foundation Mathematics (4)
- JEE Advanced Physics (3)
- JEE Main (3)
- JEE Main Chemistry (7)
- JEE Main Physics (29)
- JEE Mock Test Physics (1)
- JEE Study Material (1)
- JEE/NEET Physics (6)
- Latest Updates (13)
- CBSE (1)
- CBSE Syllabus (1)
- Maths Articles (2)
- NCERT Books (3)
- NEET Chemistry (13)
- NEET Physics (55)
- NTSE (1)
- Physics (1)
- Physics Articles (21)
- Alternating Current (1)
- Electrostatics (6)
- Fluid Mechanics (2)
- PowerPoint Presentations (13)
- Previous Years Question Paper (3)
- Products (62)
- Products for CBSE Class 10 (15)
- Products for CBSE Class 11 (10)
- Products for CBSE Class 12 (6)
- Products for CBSE Class 6 (2)
- Products for CBSE Class 7 (5)
- Products for CBSE Class 8 (1)
- Products for CBSE Class 9 (3)
- Products for Commerce (3)
- Products for Foundation Courses (2)
- Products for JEE Main & Advanced (10)
- Products for NEET (6)
- Products for ICSE (4)
- Question Answer (3)
- Topic Wise Study Notes (Physics) (2)
- Topicwise MCQs (2)
- Uncategorized (138)
Test Series (Engineering, Medical and School Level Exams)
Test series for students preparing for Engineering & Medical Entrance Exams are available. We also provide test series for School Level Exams. Tests for students studying in CBSE, ICSE or any state board are available here. Just click on the link and start test.
Download CBSE Books
Download CBSE Class 10 Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
Download CBSE Class 12 Books
Exam Special Series:
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
Download ICSE Class 10 Books
Exam Special Series:
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)

